Better Water Treatment for the Oil & Gas Industry
Chlorine dioxide improves oil recovery, increases well production, and boosts frac on the fly
Chlorine Dioxide’s (ClO2) versatility makes it the most effective and economical choice for oil and gas water treatment. Since the 1990s, International Dioxcide’s work in the oil and gas industry has proven that ClO2 offers greater microbial control (99.9%) and less corrosivity than alternative water treatments using chlorine, peroxide, bleach or ozone. Furthermore, chlorine dioxide produces significant savings by reducing treatment, logistical, and freshwater sourcing costs.
Improve Oil Recovery
Our decades of experience and independent testing have shown that chlorine dioxide water treatment can increase skim oil recovery up to 30 percent. Why? Because chlorine dioxide eliminates 99.9% of bacteria and the resulting iron sulfide and hydrogen sulfide (H2S) that cause significant emulsion. With chlorine dioxide, less emulsion means increased oil recovery and revenue. Chlorine dioxide destroys H2S by killing the sulfate reducing bacteria that produces it and oxidizing it to a sulfate.

This image shows a bottle (right) with emulsion created by iron sulfide and hydrogen sulfide, along with another showing the treatment impact of chlorine dioxide.
Increase Production Rate
Another major benefit of chlorine dioxide water treatment is increased well production. In fact, chlorine dioxide can increase well injectivity by more than 150%. Chlorine dioxide achieves this by eliminating the oxidizable compounds and biofilm that “plug” saltwater disposal or injection well operations. ClO2 eliminates 99.9% of bacteria and the resulting iron sulfide and hydrogen sulfide (H2S) that cause significant emulsion and fouling.
Other benefits:
- Restores frac wells to their original production rates
- Removes iron sulfide contaminated wellbore damage
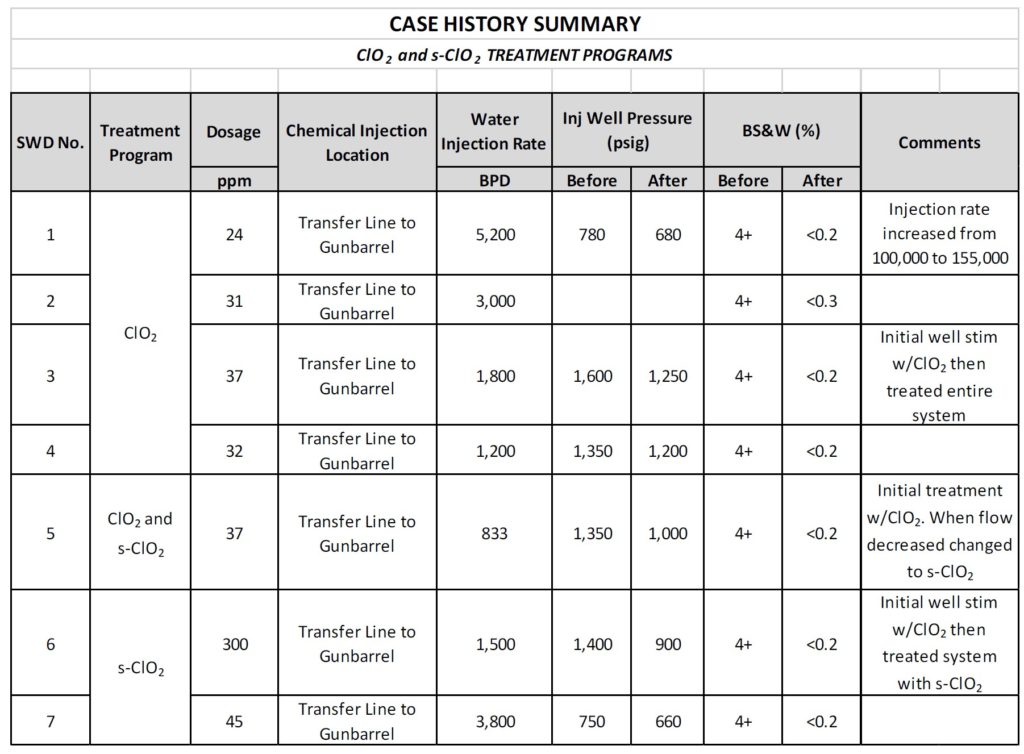
Save on Frac Water Treatment
In addition to improved well production and oil recovery, chlorine dioxide can reduce frac water treatment costs by 20% or more. Chlorine dioxide eliminates 99.9% of acid-producing and sulfate-reducing bacteria and reduces bottle turns to zero━and it achieves this at a lower dose compared to other biocides.
Other benefits:
- Reduces truck traffic by 50% or more
- Reduces frac well freshwater sourcing costs by up to 90%
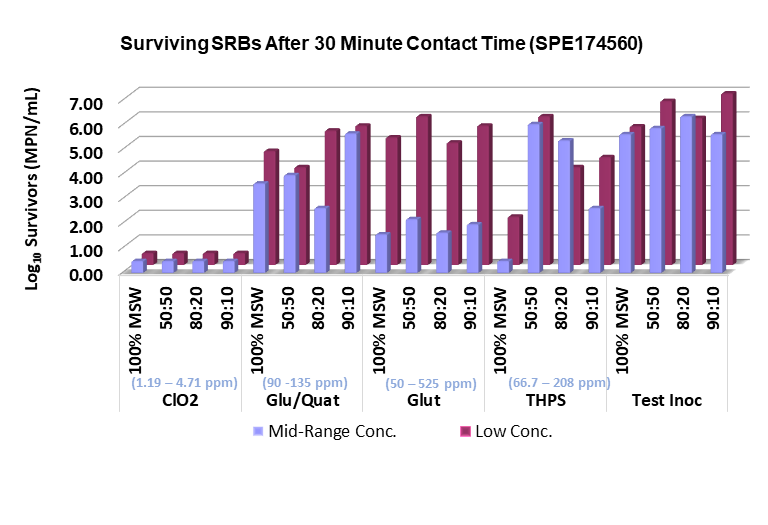
Above: The performance of chlorine dioxide in eliminating sulfate-reducing bacteria (SRBs) is compared to alternative methods used in frac water treatment.
Below: Similar tests are performed to measure the performance of chlorine dioxide at Eagle Ford and Marcellus Shale frac sites.
Marcellus Treatment Results
|
Individual Frac Stage Cell Counts
APB & SRB |
Bottle Turns APB | Bottle Turns SRB | |
|---|---|---|---|
| No Treatment | 100-1,000/ml | 3 | 2 |
| 10,000/ml | 4 | 4 | |
| 100-1,000/ml | 2 | 4 | |
| 1,000-10,000/ml | 4 | 3 | |
| ClO2 Treatment | 0-10/ml | 1 | 0 |
| 0/ml | 0 | 0 | |
| 0/ml | 0 | 0 | |
| 0/ml | 0 | 0 | |
| 0/ml | 0 | 0 | |
| 0/ml | 0 | 0 | |
| 0/ml | 0 | 0 | |
| 0/ml | 0 | 0 | |
| 0/ml | 0 | 0 |
Eagleford Treatment Results
| Average Frac Stage Cell Counts APB & SRB | Bottle Turns | |
|---|---|---|
| No Treatment | 1,000-100,000/ml | 3-5 |
| Post Non-Oxidizing Biocidee | <100/ml | 1-2 |
| Post ClO2 Treatment | <10/ml | 0-1 |
Oil & Gas Water Treatment Partner of Choice
International Dioxide’s chlorine dioxide equipment and technical expertise has been put to work on over 1,500 oil and gas wells throughout the Bakken, Barnett, Eagle Ford, Marcellus, Permian, Piceance, Utica, and Woodford Basins. Our relationships with leading field service companies forms the largest network of ClO2 water treatment in North America.