Chlorine Dioxide Chemistry FAQs
ClO2 is a powerful disinfectant that reacts rapidly via oxidation to provide effective microbiocidal impact. It delivers broad spectrum performance against bacteria, fungi, algae, viruses, and parasitic microorganisms. ClO2 kills vegetative microorganisms and effectively deactivates sporulated species. By holding a minimal, easily detectable ClO2 residual concentration for a period, vegetative bacteria are killed quickly at low effective dose levels, and sporulates species inactivation occurs at low concentration time values, CT.
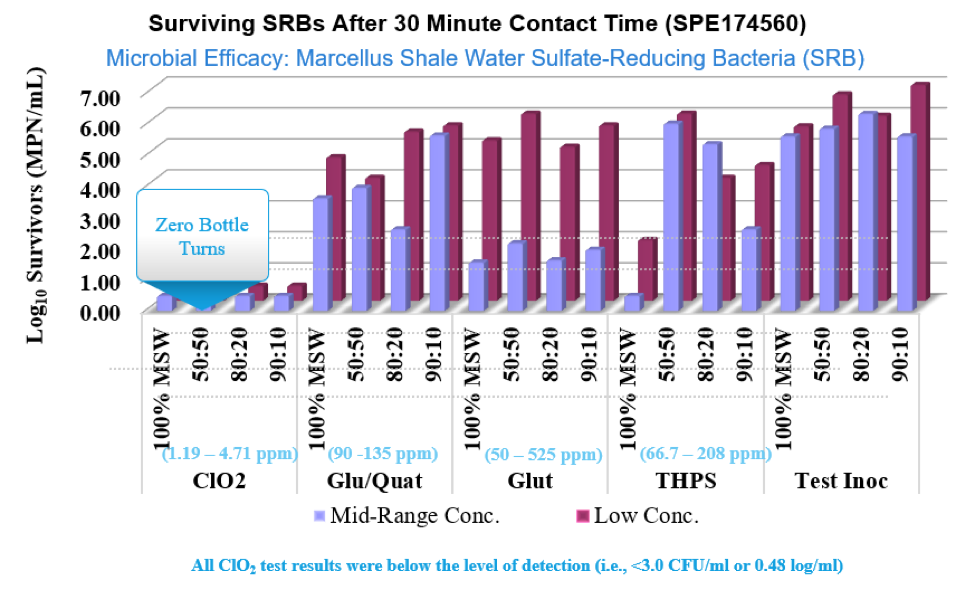
ClO2 rapidly inactivates waterborne viruses like Rotavirus and can also be used to kill both Giardia cysts and Cryptosporidium oocysts. In industrial applications it is very effective against Sulfate Reducing Bacteria, SRB’s, and Acid Producing Bacteria, APB’s.
In its primary role as a disinfectant, ClO2 disrupts critical cell physiological functions, such as protein synthesis and cell membrane permeability. As a dissolved gas, ClO2 can diffuse through the cell membrane and react selectively with cellular components, such as amino acids (cysteine, tryptophan and tyrosine), viral capsids, and assembly proteins. The oxidation mechanism targets disulfide bonds in amino acids, and this results in damage to the tertiary and quaternary protein structures as well as alteration of the outer cell membrane. The collective damage that results from this multi-faceted cellular attack makes ClO2 both broad spectrum and efficacious at very low dose rates.
Being an oxidizer, ClO2 also readily oxidizes chemicals such as sulfide, ferrous iron, dissolved manganese ions, and organic molecules with electron-rich groups. However, many organic molecules are not easily oxidized by ClO2. Consequently, chlorine dioxide minimizes wasteful reactions with organic molecules in contrast to more aggressive, less selective oxidizers. This conserves ClO2 for its intended disinfection and/or targeted oxidation purposes, thus promoting lower application dosages and lower generation of toxic by-products versus other non-in-kind chemistries.
View this animated illustration showing how chlorine dioxide is a highly selective oxidizer
Chlorine dioxide is one of four U.S. EPA disinfectants approved for potable water use. When applied at the appropriate concentrations, chlorine dioxide does not form any appreciable halogenated byproducts, and the ClO2 residual byproducts are typically within the recommended EPA limits.
Chlorine dioxide kills bacteria through multiple mechanisms. In bacteria, chlorine dioxide oxidizes fatty acids and membrane lipids in the cell membrane. This disrupts the permeability of the outer membrane, resulting in the inability of the cell to regulate the movement of molecules into and out of the cell. ClO2 also denatures cellular proteins in the membrane and within the cell itself by oxidizing amino acids that make up these proteins. As a result, the proteins change shape and lose functionality. This can make the cell unable to generate energy, unable to move molecules in and out of the cell and unable to stay alive.
In bacterial endospores, the inner membrane is essential for viability. ClO2 penetrates and damages the inner membrane making the spore leaky. In viruses, as shown in this RNA virus, ClO2 reacts with the protein capsid allowing RNA to be released. The RNA then reacts with the ClO2 and RNA synthesis is disabled, so the virus cannot replicate. These reactions with cellular biomolecules result in impairment or death of the microorganism. The collective damage from this multifaceted attack prevents the microorganism from developing resistance and makes ClO2 both broad spectrum and efficacious at a low dose.
In addition to its oxidative selectivity, chlorine dioxide has three more distinct advantages over chlorine as an oxidizing biocide.
First, because ClO2 does not undergo hydrolysis in water, but remains a dissolved gas, it is able to maintain its oxidative properties over a broad pH range from various acidic environments below pH 4 to basic environments of pH 10. Chlorine, on the other hand, has a narrow pH efficacy range and is a relatively ineffective antimicrobial in alkaline environments.
Second, ClO2 is a five electron oxidizer and on a per weight basis provides 2.6 times the oxidative capacity of chlorine.
Third, ClO2 does not chlorinate organic molecules as does chlorine. Chlorine can react by addition or substitution reactions, which incorporates a chlorine atom into an organic compound. This leads to the formation of harmful chlorinated disinfection byproducts such as trihalomethanes (THMs), chloroform, and dioxins. Compared to other oxidizing biocides, all these attributes make chlorine dioxide a very effective and efficient choice for disinfection.
View this animated illustration to understand three distinct advantages that chlorine dioxide has over chlorine in water disinfection.
In more contaminated water systems, ClO2 works well reducing bio-fouling and providing superior water disinfection properties. Generally, the higher the contaminant loading the greater the economic advantage over alternative chemistries due to its more selective oxidation reactivity.
In wastewater treatment applications, ClO2 is used to disinfect treated water prior to discharge to minimize chlorinated organic formation which is limited by permit. ClO2 is also used to oxidize hydrogen sulfide odors and minimize potential corrosion concerns at the head works, sludge press and collection systems.
For oil and gas applications,ClO2 allows completion and production engineers a real time, verifiable, solution to control aerobic and anaerobic bacteria during surface operations, fracturing and downhole interventions. ClO2 is effective on SRB’s, APB’s and other species, spore and oocysts. Additionally it oxidizes both hydrogen sulfide and iron sulfide which stabilize emulsions and create exposure, corrosion, blockage and quality concerns.
In cooling applications, ClO2 is used in very large industrial scale cooling towers where contamination makes other biocide programs ineffective or uneconomical. It is also used in hospital and healthcare environments as a sanitization/sterilization agent and for Legionella control.
ClO2 chemistry is well established technology that is currently being used in a wide variety of industrial, oil and gas, food and municipal applications all around the globe. As the use of traditional chlorine, bleach or other biocides which produce disinfection byproducts continues to be subject to more stringent regulatory compliance requirements, ClO2 is being specified as a replacement in cutting-edge applications.
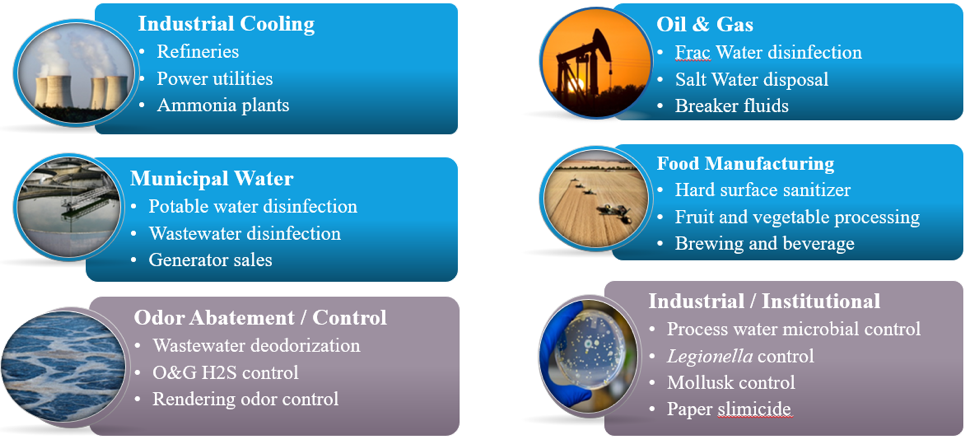
Applications where ClO2 excels:
- Ammonia plants
- Aquifer storage and recovery (ASR)
- Brewing and beverage
- Cooling tower
- Ethanol fermentation
- Flume water
- Fruit and vegetable processing
- Hard surface sanitizer
- Industrial process waters
- Leather bleaching
- Legionella control
- Mollusk control
- NOx oxidation
- Odor abatement/control
- Oil and gas hydraulic fracturing
- Paper slimicide
- Potable water disinfections
- Power utilities
- Process waters microbial control
- Pulp bleaching
- Rendering odor control
- RO membranes (RO/NF/UF/MF)
- SOx oxidation
- Wastewater deodorization
- Wastewater oxidation
- Zebra mussel control
Surviving microorganisms are known to alter their structure to develop immunity to conventional biocides. In such cases continuing treatment requires a shock with higher and higher dosages or alternating biocidal chemistries. This is not known to occur with ClO2 treatment.
Chlorine dioxide is different from traditional oxidizers such as hypochlorite and other oxidizers that are ionized molecules in solution. Chlorine dioxide is a dissolved gas in solution. Therefore, it easily penetrates polysaccharide biofilms and the microbial cell wall via diffusion and performs its oxidative function on the metabolic biochemical components of the microbe. Oxidizers that hit multiple targets are very rapid and effective microbial control agents. With chlorine dioxide, the metabolic rate of the microbial species is vastly less important, and the rate of effectiveness is considerably more uniform.
ClO2 reacts with the basic cell structure, and shuts down the metabolism of microorganisms, rendering them incapable of modifying their cell structure and, therefore, making the use of alternating chemistries unnecessary.
Chlorine dioxide destabilizes biofilm formations and provides ongoing control of biofilm regrowth. Biofilms are known to reduce flow, increase pressure, and provide shelter for sulfate reducing bacteria (SRBs) and acid producing bacteria (APBs). These bacteria are known to cause significant corrosion in piping and fluid handling systems.
As a true dissolved gas-in-solution, ClO2 rapidly penetrates into the biofilm substrate, delivering biocidal effectiveness throughout and beneath the biofilm, essentially eliminating underlying APBs and SRBs where they live. In addition, as chlorine dioxide reacts as a biocide, chlorite ion is formed as a ClO2 disinfection byproduct. When chlorite ion encounters acids, such as those produced by SRBs and APBs, chlorine dioxide is reformed in situ. In effect, a cycle of chlorine dioxide consumption to chlorite ion, and a partial regeneration back to chlorine dioxide, is possible in many biocidal applications, including biofilm control.
ClO2 is a well-recognized biocide treatment for cooling water and process water applications, especially when biofilm removal and inhibition is the goal.
View this animated illustration showing chlorine dioxide’s effectiveness in eliminating and preventing biofilm formations.
No. Although chlorine is part of the chemical structure of ClO2, a key chemical attribute of ClO2 reaction chemistry is that it does not produce halogenated DBPs from the oxidized compound. ClO2 only reacts with substances that give up electrons in true redox reactions. Therefore, ClO2 functions as a highly selective oxidant when it reacts with organic compounds, because it only attacks electron-rich bonds in the organic compounds. In true oxidation the organic give up electrons to the chlorine dioxide molecule.
In contrast, because pH impacts the reaction chemistry of chlorine, it can attach a chlorine atom or substitute a chlorine atom into an organic compound, which leads to the formation of undesirable chlorinated organics, such as chloroform, bromoform, dioxins or THMs. These halogenated DBPs are coming under increasing regulatory pressure around the globe.
The predictable redox reaction chemistry of ClO2 with organics is also important, because it significantly reduces the formation potential of halo acetic acid precursors, such as aldehydes, ketones and ketoacids, which then lowers the potential for THM formation in finished treated drinking water.
ClO2 gas is highly soluble in water and does not hydrolyze into ions. This enables it to maintain its oxidative and biocidal properties over a broad pH range—from 4 to 10. This characteristic is contrary to traditional oxidizing biocides (chlorine and bromine), which have a narrow pH efficacy range and are relatively ineffective antimicrobials in alkaline pH environments (see Figure 2).
Oxidative capacity refers to the amount of electrons that can be transferred to a given oxidizer. A single ClO2 molecule can accommodate up to five electrons, which gives it 2.6 times the specific oxidative capacity of chlorine, which can only accept 2 electrons.
Oxidizer selectivity refers to the variety of chemical species with which the oxidizer readily reacts. In the case of a more selective oxidizer, a smaller array of redox reactions will occur. The strength of a given chemical to act as an oxidizer is represented by the standard reduction potential, E°, which is measured in volts. For ClO2, E° = 0.95 V, which makes it a less aggressive oxidizer compared to many other common disinfectants used in water treatment applications, such as ozone at 2.07 V and hypochlorous acid at 1.49 V. These fundamental properties make ClO2 a very efficient oxidizing disinfectant.
View this animated illustration showing chlorine dioxide’s oxidizing potential.
Chlorine dioxide and its reaction products eventually return to sodium chloride, salt. ClO2, chlorate (ClO3–) and chlorite (ClO2–) can all eventually decompose into chloride ion and typically be re-associated with earth mineral cations to form simple salts (NaCl). In the case of ClO2, the first reaction takes up an electron and reduces to chlorite ion:
ClO2 + R(e-) → ClO2–
The chlorite ion is then available for further redox reactions, including environmental reducing agents, photodecomposition and biochemical uptake by bacterium, becoming a chloride ion:
ClO2– + 4 R(e-) → Cl– +O2
These reactions illustrate that ClO2 can be reduced to chloride ion, and, that during this reaction process, it accepts five electrons. Chloride ion is the most ubiquitous ion in the earth’s environment. A similar amount of chloride ion will be returned to the environment as administered from the ClO2 dose applied.
Sir Humphrey Davy is credited with the discovery of ClO2 in 1814. In 1944, ClO2 was first used commercially in the United States—for taste and odor control in the city of Niagara Falls, NY drinking water supply system. Twelve years later, the first large-scale ClO2 use in Europe was in the drinking water supply for the city of Brussels, Belgium.
In most industrial and municipal applications, ClO2 is generated on-site at the point of use, because it cannot be safely compressed like chlorine gas—making it difficult to transport. The majority of this ClO2 is produced in specially designed generation equipment systems, which safely combine sodium chlorite (NaClO2)—the primary registered chemical precursor for ClO2—in combination with one or two additional chemical precursors to produce ClO2 on-site.
ClO2 is safe to make and use given the proper understanding of the chemistry, safe equipment design and training support. Like many chemistries ClO2 and it’s precursors must be handled with respect and applied properly. ClO2 has been generated on-site and used safely and successfully in many different applications for many years. IDI is a leader in the safe generation and application of ClO2. With over 1000 generators installed around the world IDI brings over 70 years of expertise in applying ClO2 safely. Our vacuum based innovative equipment design ensures maximum safe operations.
The O&G partner program has been active in the field for over half a decade and prepares your workforce to safely and effectively apply ClO2. It is designed to assist service companies through concept, implementation and execution of a ClO2 treatment program. It encompasses pre-deployment (engineering, design integration, build, initial safety training), deployment (install, commission, start-up, field resource, operational training, service) and post-deployment (chemical supply, technical support, lab support, call center support, renewal training).
IDI has numerous case studies and testimonials. Here are several summaries for O&G applications and a link to a recent application paper prepared for the Southwest Petroleum Short Course:
Generally, any water to be treated will exhibit an oxidant demand profile for ClO2. Iron, Manganese and sulfur species will oxidize first more quickly than the biological components of bacteria present. Once oxidation is, to a great extent, complete a residual of ClO2 can be detected. When a consistent residual of ClO2 is established and held for a period dictated by the application, disinfection is completed. The best way to determine how much and how long to hold a residual is to perform a site-specific demand test. Simple analytical test methods are used to determine the residual ClO2 present. (Brief Demand Test Video)
It has been demonstrated that asphaltenes, inorganic fines, clays, and particularly iron sulfide particulates will stabilize oil water emulsion layers. Particularly where SRB’s are active producing hydrogen sulfide, iron in the water will combine with sulfide to for very fine iron sulfide. To improve emulsion breaking one must address both the stabilizing sulfides and the source of the sulfides. ClO2 acts as a direct oxidizer for metal sulfides, hydrogen sulfide and other inorganic fines. Oxidation allow formation of floc both soluble sulfates and floc that will settle, removing the stabilizing effect of the sulfide fines. In addition, ClO2 will act as a dissolved gas easily penetrating the emulsion, biofilm and other phases to kill the SRB’s and prevent further generation of sulfide stabilizing fines. These attributes are valuable in surface tank collection systems, salt water disposal wells, water floods, and other systems where oil water emulsions exist including resolving near well bore blockage in production and injection wells.
Inadequate microbial control can have a significant impact on downhole corrosion rates and can create both economic cost and sustainability concerns. SRBs (sulfate-reducing bacteria) are the primary cause of microbial-influced corrosion. Chlorine dioxide quickly and effectively kills SRBs and oxidizes the hydrogen sulfide (H2S) they produce, reducing the sour in the formation water that further reduces the corrosion rates. Chlorine dioxide at typical dose levels ultimately reacts to form sodium chloride at levels that are insignificant when compared to the natural salt levels found downhole. Because its oxidation potential is much lower than chlorine, peroxide, or ozone, and it does not need an extreme pH level to be an effective oxidant, use of chlorine dioxide results in lower corrosivity. It is clear the benefits of using ClO2 as a topside disinfectant in oil and gas applications are significant.
Recently published studies continue to show that ClO2, when properly generated and used at typical 1-5 ppm residual concentrations for disinfection of frac water, has minimal impact on the corrosive erosive nature of fracturing operations. With the increase in produced water recycle for fracturing, frac iron systems are seeing higher salt concentrations and just like sea water systems, they will experience wet dry cycles that allow concentration of salts which significantly increase general corrosion and pitting. Proper use of inhibitors can minimize corrosion from low pH and salts, but not eliminate it. It can be concluded from these studies that acidic solutions are the main contributor to corrosion in frac systems followed by salt from produced water.
Yes, we have numerous reference papers regarding chlorine dioxide chemistry and applications. Here is a brief list for O&G applications.
One growing application for ClO2 is in bromide-rich source waters, where oxidizers with higher oxidation-reduction potential (ORP) values will react with the bromide in the source water to form bromate, a highly toxic and strictly regulated disinfection byproduct (DBP) with US MCL = 10 µg/L. ClO2 has a lower ORP value than bromine, and, therefore, does not oxidize bromide to form bromate.
Learn more about chlorine dioxide, the highly effective biocide offered by International Dioxcide, a division of ERCO Worldwide.
We appreciate your interest in International Dioxcide. Take a moment to request additional information about our products and services.